Earth is often referred to as the 'water planet" for good reason. Water is the most abundant natural liquid on Earth and has unique physical and chemical properties that help make the planet habitable. Water plays important roles in weather and climate. Based on water's molecular weight as well as the freezing and boiling temperatures of chemically related substances, fresh water should freeze at about −90ºC (−130ºF) and boil at about −70ºC (−94ºF). Actually, fresh water's freezing point is 0ºC (32ºF) and its boiling point is 100ºC (212ºF) at average sea level air pressure. Water is one of the very few naturally occurring substances capable of existing in all three phases within the temperature and pressure ranges found at and near Earth's surface.
Water's unusual properties arise from the physical structure of the water molecule (H2O) and hydrogen bonds linking water molecules. Without hydrogen bonding, water would exist only as a gas within Earth's usual range of surface temperatures and pressures, meaning that Earth would have no water cycle, no ocean, no ice caps, and probably no life.
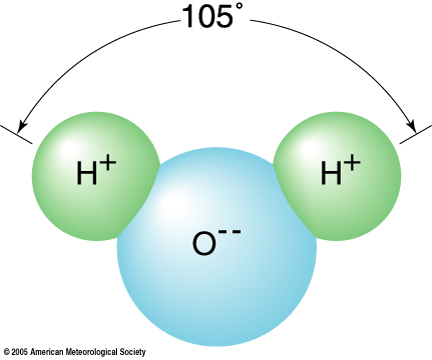
Water is a relatively simple chemical compound with three light atoms, two hydrogen (H) atoms and one oxygen (O) atom, constituting a single water molecule. Within a water molecule, the single electron from each of the two hydrogen atoms is shared with the six bonding electrons of the oxygen atom. (An electron is a negatively charged subatomic particle.) This covalent bonding is so strong that the water molecule resists dissociation into its constituent hydrogen and oxygen atoms. (As noted in Chapter 2 of the textbook, photodissociation of water is not a major source of free oxygen in the present atmosphere.) However, the electron density is not uniform about the water molecule, as the three atoms are not in a straight line but have a configuration similar to the diagram on the right since the oxygen atom having a stronger attraction for shared electrons than the hydrogen atoms. The approximate 105-degree bond angle formed by the arrangement of the hydrogen-oxygen-hydrogen atoms produces a charge separation in the water molecule. Hence, the oxygen acquires a small negative charge leaving the hydrogen with a small positive charge. Molecules having a separation of positive and negative charges are described as polar.
Opposite electrical charges attract so that, like tiny magnets, neighboring water molecules link together. The positively charged (hydrogen) pole of a water molecule attracts the negatively charged (oxygen) pole of another water molecule. This electrostatic coupling between adjacent water molecules is known as hydrogen bonding. Water molecules can form hydrogen bonds in three directions; that is, each molecule has three potential sites for hydrogen bonding. Hydrogen bonding is roughly 10 to 50 times weaker than the covalent bonds between the hydrogen and oxygen atoms of individual water molecules. Nonetheless, they are strong enough to significantly influence the physical and chemical properties of water especially in the solid and liquid phases.
Hydrogen bonding inhibits changes in water's internal energy, meaning that greater additions or losses of heat are required to change water temperature as compared to other chemically related substances. With its unusually high specific heat, water can store great quantities of heat energy thereby moderating diurnal and seasonal air temperature contrasts especially downwind from the ocean and large lakes..
In addition, the hydrogen bonding inhibits internal energy changes as water absorbs from or releases to the environment unusually great quantities of heat (called latent heat) during physical phase changes from ice to liquid to gas. Water readily changes phase, contributing to the dynamic nature of the Earth system. When water changes phase heat is either absorbed from the environment or released to the environment. Melting, evaporation, and sublimation are phase changes that absorb heat as energy is needed to break some of the hydrogen bonds. Phase changes that release heat to the surroundings are freezing, condensation, and deposition. As noted in Chapter 4, phase changes of water are important mechanisms in the transfer of heat energy between the Earth's surface and atmosphere, as well as between tropical and polar latitudes.
Water occurs on Earth in all three phases: as a crystalline solid (ice), liquid (water), and gas (water vapor). The molecular structure influences water's properties in each of these phases.
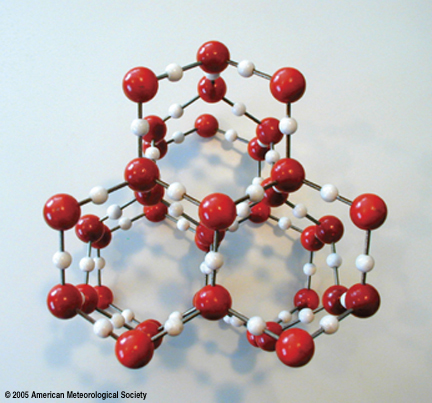
Like all crystalline solids, ice has a regular internal three-dimensional framework (crystal lattice) consisting of a repeated pattern of molecules as appearing in the accompanying picture of a ice lattice model. Each water molecule is bound tightly to its neighbors but intermolecular bonds are elastic (acting like springs) so that molecules vibrate about fixed locations in the lattice. For this reason, an ice cube retains its shape as long as the temperature is subfreezing. Hydrogen bonding is responsible for the ordered arrangement of water molecules in the crystal lattice that, in turn, explains the hexagonal (six-sided) shape of large ice crystals and snowflakes.
Most common solids sink when placed in their liquid phase-but not ice. Ice floats in liquid water. Most liquids increase in density as the temperature falls until they solidify because the molecules occupy a smaller volume with diminishing thermal agitation as they move closer together. However, the maximum density of fresh water is 1.0 g per cubic cm that occurs at about 4°C (39.2°F). As the temperature drops below 4°C, hydrogen bonding becomes dominant, causing the water to expand. As liquid water freezes to ice, the volume of the water increases by 9% as hydrogen bonding forces the water molecules into a rigid crystal structure-contrary to most other liquids that contract when they solidify. Because ice's internal framework is an open network, the molecules in ice crystals are not as closely packed as a similar number of molecules in liquid water. At 0ºC (32ºF), the density of ice is about 0.92 g per cubic cm. The density difference between water in the liquid and solid phases explains why ice floats in liquid water and why lakes freeze from the top down rather than the bottom up. In addition, expansion of ice can cause sufficient pressure to break water pipes and radiators upon freezing, unless precautions are taken.
When ice melts, it becomes liquid water. After observing ice crystals disappearing during melting, we might expect that all hydrogen bonds between water molecules would break during the transition from ice to liquid water. This is not the case. Instead, many water molecules remain linked by hydrogen bonding as transient clusters of water molecules surrounded by non-bonded (free) water molecules. Although some molecular clusters persist into the liquid phase, water molecules exhibit much greater activity in the liquid than solid phase. In the liquid phase, water molecules undergo vibrational, rotational, and translational (straight-line) motions. This greater freedom of movement explains why liquid water takes the shape of its container.
When liquid water changes to vapor, essentially all hydrogen bonds are broken. Individual molecules move about with even greater freedom than in the liquid phase, diffusing rapidly to fill the entire volume of its container. Gas molecules exhibit vibrational, rotational, and translational motion and exert a force as they bombard a solid or liquid surface. Force per unit area is defined as pressure. Water vapor molecules mix with the other components of air and contribute in a relatively small way to the total air pressure (Chapter 5).
A change in phase of water thus represents a change in molecular activity, increasing from solid to liquid to vapor. As noted above, whereas not all hydrogen bonds are broken in the transition from solid to liquid, all hydrogen bonds are broken in the transition from liquid to vapor. Hence, the magnitude of the latent heat of vaporization is more than seven times greater than the latent heat of melting.
Liquid water conducts heat energy more readily than any other liquid except mercury. This property of water coupled with convection is responsible for the relatively uniform temperatures of water bodies. Objects immersed in water cool faster in water than in air. Persons who are immersed in cold water rapidly lose body heat and quickly develop symptoms of hypothermia, a potentially lethal drop in the body's core temperature.
Except for a window near 11 micrometers, water molecules absorb and emit radiation in several broad bands within the infrared region of the electromagnetic spectrum. The absorption and emission of radiation in these bands are related to the energy associated with the vibrational motions of the three atoms in water molecule. Water in the atmosphere, whether as a vapor or as droplets or ice crystals in clouds, is Earth's principal greenhouse gas. Primarily because of water in the atmosphere, Earth's surface temperature is elevated by about 33 Celsius degrees (59 Fahrenheit degrees) making for a habitable planet.
Water is referred to as the "universal solvent" for its ability to dissolve numerous substances. The polar nature of the water molecules helps pull apart the ionic bonds of salts forming ionic aqueous solutions. In addition, hydrogen bonding facilitates the dissolving of many non-ionic substances in water. This solvent property of water plays an important role in cloud formation (Chapter 6).
URL: datastreme/learn/m_sup.html
Prepared by Edward J. Hopkins, Ph.D., email hopkins@meteor.wisc.edu
© Copyright, 2006, The American Meteorological Society.